Research Summary

Genetic engineering of plants is at the core of sustainability efforts, natural product synthesis, and agricultural crop engineering. However, the plant cell wall is often a barrier that limits the ease and throughput with which exogenous biomolecules can be delivered to plants.
Current biomolecule delivery methods to plants suffer from host range limitations, low transformation efficiencies, tissue damage or unavoidable DNA integration into the host genome, which yields a genetically-modified organism (GMO). Our lab explores the passive delivery of DNA, RNA, and protein cargoes to plants to enable high-efficiency genetic engineering of our plants and crops without foreign DNA integration. We develop these goals at the interface of nanomaterials and plant biotechnology, which is an emerging and exciting area of investigation.
The unique optical and tunable physical properties of nanomaterials can be leveraged to overcome the limitations of traditional imaging and delivery methods for plant systems.
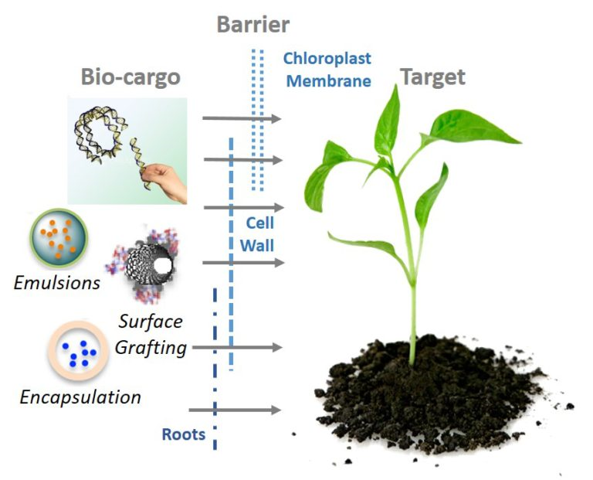
.
Carbon Nanotube (CNT) Based Delivery
We have developed an efficient diffusion-based biomolecule delivery platform for genetic transformation of several agriculturally-relevant plant species with a suite of pristine and chemically-functionalized carbon nanotubes. Efficient DNA delivery and strong protein expression without transgene integration is accomplished in mature Nicotiana benthamiana, Eruca sativa (arugula), Triticum aestivum (wheat) and Gossypium hirsutum (cotton) leaves and protoplasts.
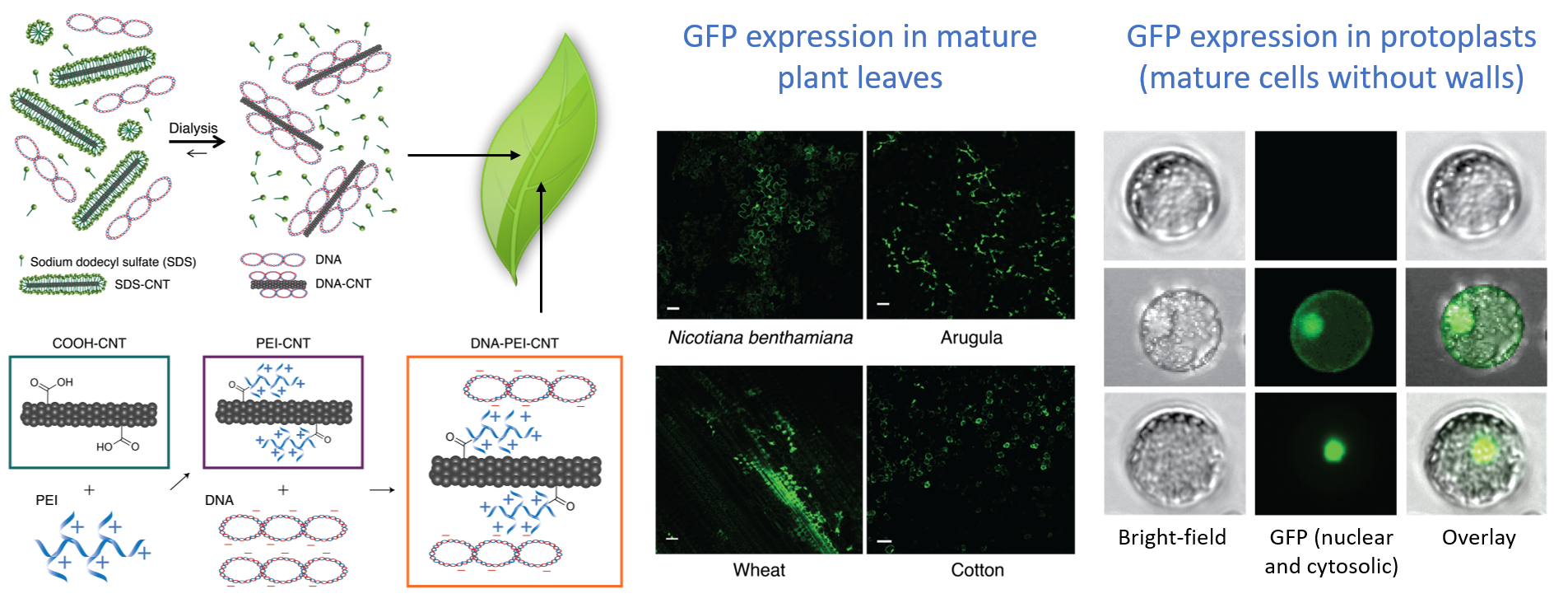
We also demonstrate a second strategy in which small interfering RNA (siRNA) is delivered to mature Nicotiana benthamiana leaves with CNTs and effectively silence a gene with 95% efficiency.
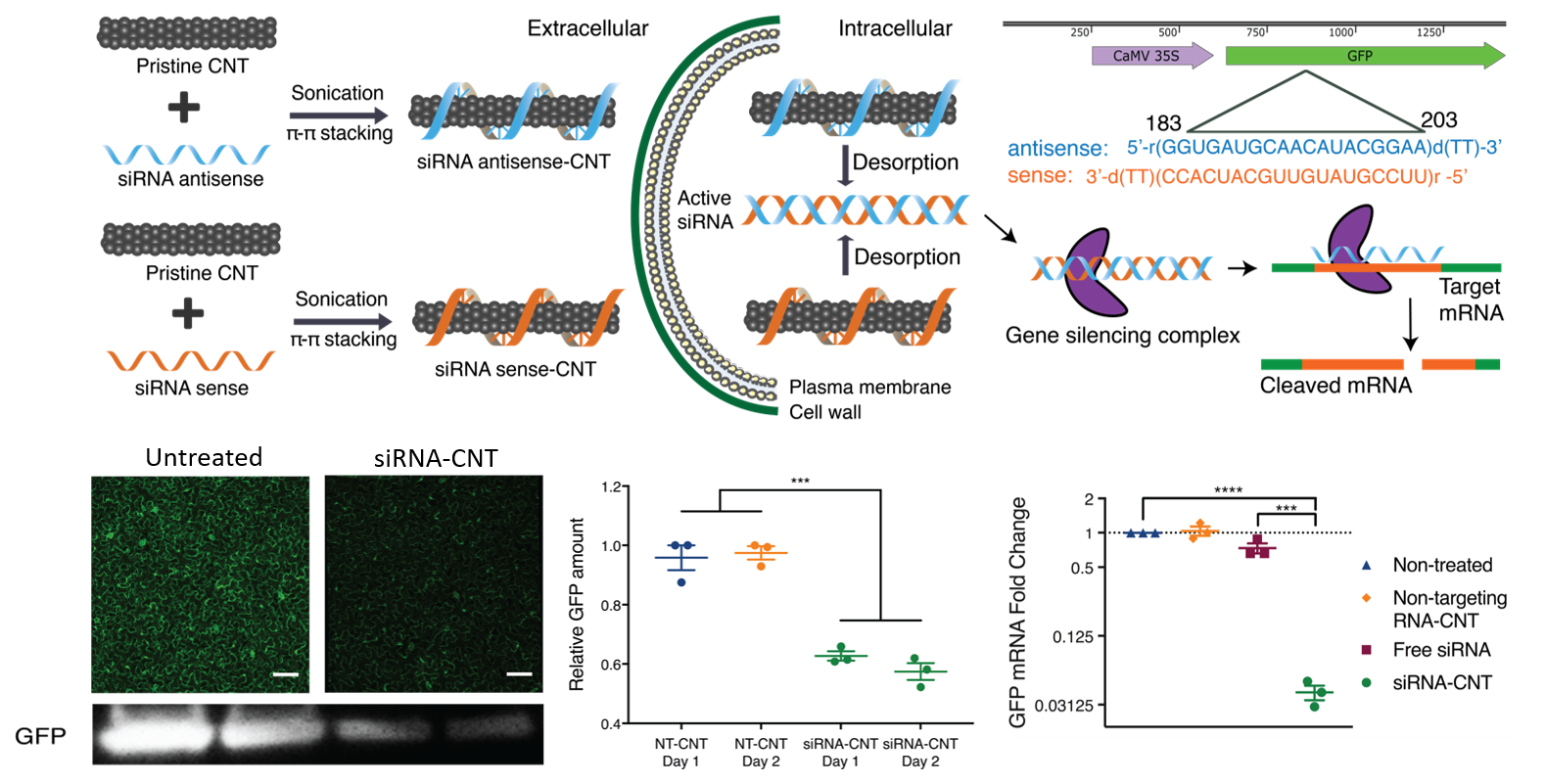
Our work provides a tool for species-independent, targeted, and passive delivery of genetic material, without transgene integration, into plant cells for diverse plant biotechnology applications (e.g. CRISPR/Cas9 editing)
.
DNA Nanostructure Based Delivery
We use DNA origami and DNA nanostructures to study the transport phenomena of nanoparticles in plant tissues and into plant cells, with a specific focus on transport across the plant cell wall. These studies systematically assess the impact of nanoparticle geometry, size, and tensile strength on in-plant transport, with proof-of-principle demonstration of their ability to deliver siRNA molecules to mature plant leaves.
To these ends, we synthesized three unique DNA nanostructures with programmed sizes and shapes: a small zero dimensional tetrahedron structure, a one dimensional tile-hairpin monomer (TH), and a one dimensional high aspect ratio nanostring.
Each nanostructure can be programmed to attach siRNA molecules to unique loci along the nanostructure, whereby the appended siRNA targets the silencing of a GFP gene.
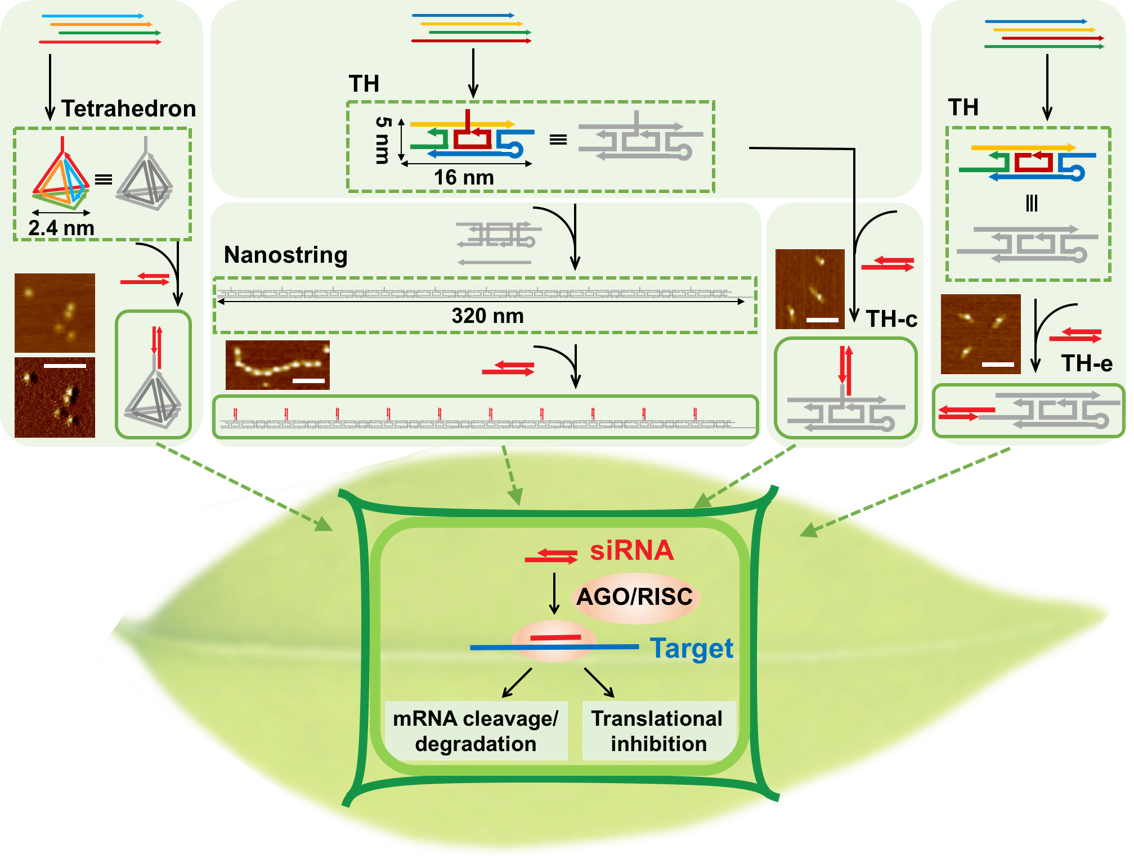
Untreated control leaves of GFP Benthamiana show strong GFP fluorescence, as expected, due to the constitutive expression of GFP in the transgenic plant. Conversely, leaves infiltrated with siRNA functionalized DNA nanostructures showed varying degrees of reduced GFP fluorescence.
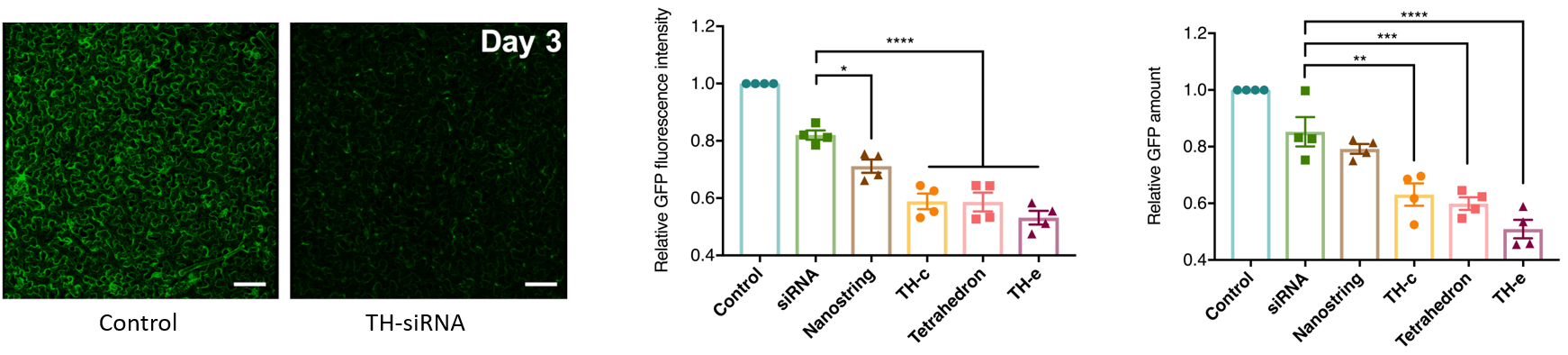
We also tested the transience of the nanostructure-enabled siRNA mediated silencing. Confocal imaging and western plot reveal that GFP levels for all leaves are recovered to the same level as non-infiltrated leaves by the 7th day following the siRNA-nanostructure infiltrations.
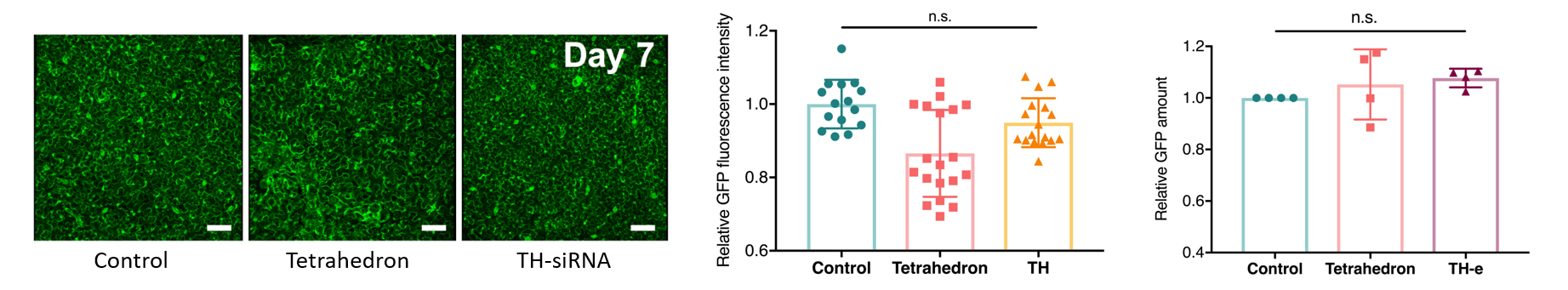
This study is important because it highlights the features of merit important for nanoparticle transport in plants. Specifically, this study demonstrates that particle size below 20 nm, and also high nanoparticle stiffness, are both features that together enable better nanoparticle transport through plant tissues and into plant cells.
.
See press coverage of our work interfacing nanomaterials with plants:
.
.
.
.
.
Scientists: Frankie Cunningham, Gabriel Dorlhiac, Natalie Goh, Eduardo Grandio, Josh Hubbard, Chris Jackson, Alison Lui, Juliana Matos, Shoichi Nishitani, Henry Squire, Liz Voke, Jeff Wang.
Select Publications: Demirer et al. Nature Nanotechnology (2019) || Zhang et al. PNAS (2019) || Demirer et al. Nature Protocols 2019 || Landry et al. Nature Nanotechnology 2019 || Demirer et al. Science Advances 2020 || Zhang et al. Nature Protocols 2020 || Demirer et al. Nature Nanotechnology 2021 || Goh et al. Nature Nanotechnology 2022






